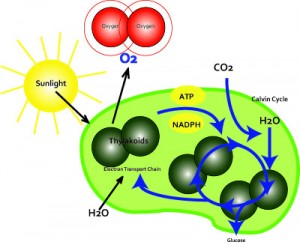
Photosynthesis:Photosynthesis uses sunlight to split water and carbon dioxide from oxygen. It is the main source of energy for nearly all forms of life on Earth. The photosynthesis of water occurs in the thylakoids of chloroplasts in plants. Thylakoids are located in the stroma. The thylakoids are flattened disks, bounded by a membrane with a lumen or thylakoid space within it. The site of photosynthesis is the thylakoid membrane, which contains integral and peripheral membrane protein complexes, including chlorophyll, which form the photosystems. One molecule of chlorophyll absorbs one photon and loses one electron. This electron is passed to a modified form of chlorophyll called pheophytin, which passes the electron to a quinone molecule, allowing the start of a flow of electrons down an electron transport chain that leads to the ultimate reduction of NADP to NADPH. This causes a formation of a proton gradient across the thylakoid membrane;which is used to synthesize photophosphorylation and the coupling of the absorption of light energy and oxidation of water to the creation of chemical energy during photosynthesis. The chlorophyll molecule regains the lost electron from the oxidized water molecule through a process called photolysis, which releases the dioxygen (O2) molecule.
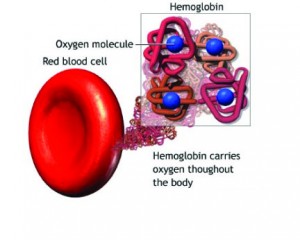
Oxygen in the Blood-Hemoglobin:
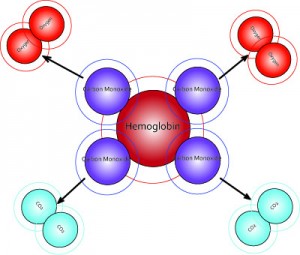
Molecular dioxygen, O2, is essential for cellular respiration in all aerobic organisms. Oxygen is used in mitochondria to help generate adenosine triphosphate (ATP) during oxidative phosphorylation. The reaction for aerobic respiration is essentially the reverse of photosynthesis. In vertebrates, O2 diffuses through membranes in the lungs and into red blood cells. Hemoglobin binds O2, changing its color from bluish red to bright red. Hemoglobin in the blood carries oxygen from the respiratory organs (lungs or gills) to the rest of the body tissues where it releases the oxygen to burn nutrients to provide energy to power the functions of the organism, and collects the resultant carbon dioxide to bring it back to the respiratory organs to be dispensed from the organism. The hemoglobin molecule can carry up to four O2 oxygen molecules. The hemoglobin releases its oxygen molecules and replaces them with carbon dioxide molecules and carries the carbon dioxide back to the lungs where the exchange repeats.
Oxygen in the Body Tissues-Myoglobin: Myoglobin is an iron and oxygen binding protein found in the muscle fibers of most vertebrates and almost all mammals. It is very similar to hemoglobin in structure and sequence, but is not a tetramer; instead, it is a monomer that lacks cooperative binding. It is used to store oxygen rather than transport it. High concentrations of myoglobin in muscle cells allow organisms to hold their breaths longer.During periods of oxygen deprivation oxymyoglobin releases its bound oxygen which is then used for metabolic purposes. Each myoglobin molecule contains one heme prosthetic group inserted into a hydrophobic cleft in the protein. Each heme residue contains one central coordinately bound iron atom that is normally in the Fe2+, or ferrous, oxidation state. The oxygen carried by hemeproteins is bound directly to the ferrous iron atom of the heme prosthetic group.
Hemocyanin and Hemerythrin: Hemocyanins are respiratory proteins in the form of metalloproteins containing two copper atoms that reversibly bind a single oxygen molecule (O2). Hemocyanins are not bound to blood cells but are instead suspended directly in the hemolymph. The copper atoms of hemocyanin are bound as prosthetic groups coordinated by histidine residues. Hemerythrin is an oligomeric protein responsible for oxygen (O2) transport in the marine invertebrate phyla of sipunculids, priapulids, brachiopods, and in a single annelid worm, magelona. It is a monomeric O2-binding protein found in the muscles of marine invertebrates. Most O2 carriers operate via formation of Dioxygen complexes, but hemerythrin holds the O2 as a hydroperoxide. The site that binds O2 consists of a pair of iron centres. The iron atoms are bound to the protein through the carboxylate side chains of a glutamate and aspartates and through five histidine residues. The uptake of O2 by hemerythrin is accompanied by a two-electron oxidation of the diferrous centre to produce OOH- complex.
Carbon Monoxide and Other Expellants: Carbon monoxide binds more strongly with hemoglobin and myoglobin than oxygen or carbon dioxide, forming a tight bond (the compound carboxyhemoglobin) that blocks hemoglobin from binding with either. Carbon monoxide begins to cause symptoms of oxygen deprivation when its blood concentration reaches 10 percent, impairs neurologic function at 30 percent, and can cause death at 50 percent. A gas commonly present in the environment, carbon monoxide is a byproduct of incomplete combustion. There is also competitive binding affinity for cyanide (CN-), sulfur monoxide (SO), nitric oxide (NO), and sulfide (S2-), including hydrogen sulfide (H2S). All of these bind to iron in heme without changing its oxidation state, but they nevertheless inhibit oxygen-binding. Pollutants are a large inhibitor of oxygen in the atmosphere. Their affinity reduces the production of oxygen molecules.